Usually they are metal oxides that is compounds of metallic elements and oxygen but many ceramics.
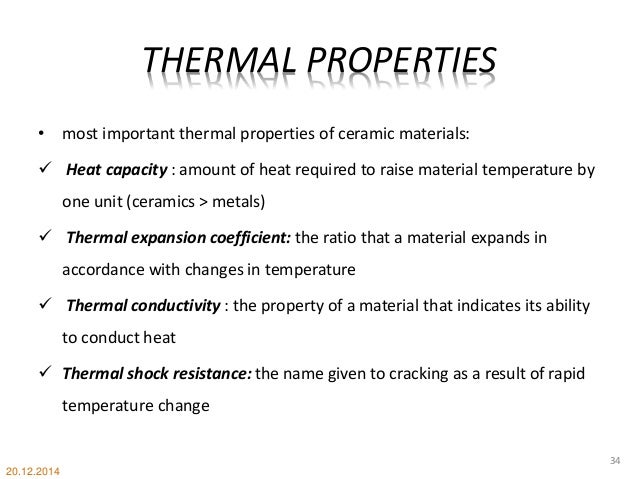
Important properties of ceramic materials.
Ceramic materials can be identified by their general properties like high hardness brittleness chemical stability and low thermal conductivity.
Most ceramics are made up of two or more elements.
People first started making ceramics thousands of years ago pottery glass and brick are among the oldest human invented materials and we re still designing brand new ceramic materials today things like catalytic converters for today s cars and high temperature superconductors for tomorrow s computers.
The density of ceramics is intermediate between polymers and metals.
There s quite a big difference between age old general purpose.
The properties of ceramic materials like all materials are dictated by the types of atoms present the types of bonding between the atoms and the way the atoms are packed together.
Generally ceramic particles are fine and coarse.
Advanced ceramics and traditional ceramics are the main categories of ceramic materials.
Industrial ceramics are commonly understood to be all industrially used materials that are inorganic nonmetallic solids.
They withstand chemical erosion that occurs in other materials subjected to acidic or caustic environments.
This is known as the atomic scale structure.
Crystalline materials have high density than non crystalline materials.
What is a ceramic.
Some elements such as carbon or silicon may be considered ceramics ceramic materials are brittle hard strong in compression and weak in shearing and tension.
A ceramic material is an inorganic non metallic often crystalline oxide nitride or carbide material.
We determine the above all properties with the particle sizes of the material.
These material properties are utilized to produce number of commercial and domestic products such as pottery bricks advanced functional items etc.
Ceramics are compounds of metals and non metals.
These are very important parameters for the ceramic material.